Bronze, as one of the earliest alloys used by mankind, has a story deeply intertwined with the progress of human civilization. Around 3000 BC, humanity entered the “Bronze Age,” marking the transition from the Stone Age to the era of metal tools. Bronze artifacts, with their excellent hardness, durability, and castability, were widely used in manufacturing weapons, tools, ceremonial objects, and art, completely transforming human production and lifestyle.
What is Bronze

From a professional standpoint, bronze is an alloy primarily composed of copper. Generally, bronze alloys have a copper content that ranges from 85% to 95%, while their core alloying elements (such as tin, aluminum, and silicon) make up 5% to 15%. This precise ratio is what gives bronze its superior hardness, strength, and corrosion resistance compared to pure copper.
How Bronze Is Made
The creation of bronze is a precise metallurgical process at its core. It involves melting and mixing two or more metals in specific proportions to achieve a new material with properties superior to those of the individual components. This process typically involves a few key steps:
1. Raw Material Preparation First, engineers accurately weigh the copper and the primary alloying element—tin or another metal—based on the desired bronze type. The copper used is generally of high purity (99.9% or higher) to ensure the quality of the final alloy.
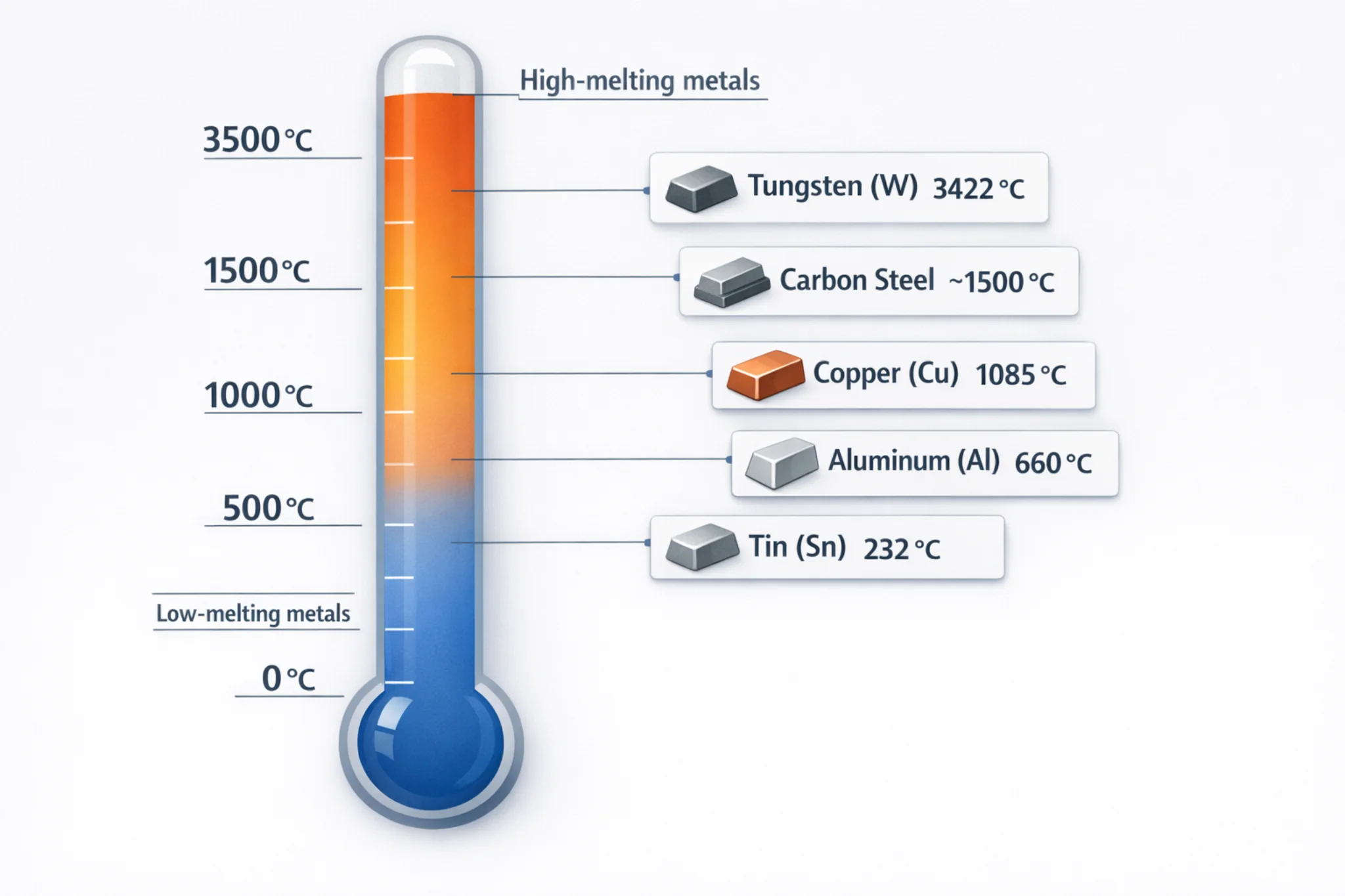
2. Melting and Alloying The raw materials are placed into a furnace and heated. Since copper has a high melting point (1083∘C) while tin’s is much lower (232∘C), the copper is usually melted first. With precise temperature control, the tin or other alloying elements (like aluminum or silicon) are then added to the molten copper and thoroughly stirred to ensure a uniform mixture.
3. Cooling and Solidification Once the mixture achieves the desired uniformity, the liquid bronze is poured into a mold. As it cools and solidifies, the copper and tin atoms form a stable solid solution. This structural change is what gives the new alloy its superior hardness, strength, and corrosion resistance compared to pure copper.
Characteristics of Bronze
Bronze stands out among many metal materials due to its unique combination of properties. These characteristics make it exceptionally well-suited for specific applications:
- Exceptional Corrosion Resistance: Bronze performs exceptionally well in humid, saltwater, or marine environments. When its surface is exposed to air, it forms a tough, stable film of oxides (patina). This layer effectively prevents deeper oxidation, protecting the metal’s interior from further corrosion.
- High Strength and Wear Resistance: The alloying process gives bronze significantly higher hardness and tensile strength than pure copper. This characteristic allows it to maintain its structural integrity even under heavy loads and high friction, making it an ideal material for manufacturing critical mechanical components like bearings and gears.
- Good Castability: Bronze has a relatively low melting point and excellent fluidity in its molten state. This makes it easy to cast into complex, precise shapes, making it a top choice for manufacturing high-precision parts and artistic pieces.
- Non-Magnetic Properties: Bronze is not magnetic. This characteristic makes it especially important for specific applications where magnetic interference must be avoided, such as in compasses, sensitive instruments, and certain marine equipment.
- Unique Color and Luster: Bronze typically presents a golden or reddish-yellow hue. Over time, with exposure and oxidation, it gradually forms a distinct dark brown or greenish-blue patina, giving it a classic and elegant aesthetic. This color change is highly valued in bronze sculptures and architectural decorations.
- Excellent Acoustic Properties: Bronze possesses superior resonance and damping capabilities. When struck, it can produce a clear, sustained resonant sound, making it an ideal material for manufacturing musical instruments like bells and cymbals.
Physical Properties of Bronze
In addition to the characteristics above, bronze also has several key physical properties that directly influence its performance in engineering applications:
- Melting Point: The melting point of bronze typically ranges from 900∘C to 1050∘C. The specific melting point depends on its alloy composition; higher tin content usually results in a lower melting point.
- Density: The density of bronze is typically between 7.4g/cm3 and 8.9g/cm3. This is slightly lighter than or similar to steel (7.85g/cm3) but much heavier than aluminum (2.7g/cm3), which contributes to its high load-bearing capacity and substantial feel.
- Electrical Conductivity: Compared to pure copper, bronze has significantly lower electrical conductivity. This makes it unsuitable for conductive applications, but its low conductivity and non-magnetic nature give it an advantage in certain specialized electronic or sensor applications.
Disadvantages of Bronze
Although bronze has significant advantages, it also has some limitations:
- High Cost: Due to the price of its main components, copper and tin, bronze is often much more expensive than other common metals like carbon steel and cast iron.
- Heavy Weight: Bronze’s high density makes it heavier than lightweight alloys like aluminum, making it unsuitable for applications where weight is a critical factor.
- Prone to Discoloration in Certain Environments: While it is highly corrosion-resistant, bronze can oxidize and form a patina in certain chemical environments, which may affect its aesthetic appeal.
Main Types of Bronze
Based on the primary alloying elements used, bronze can be classified into several main types, each with unique properties and a specific composition.
- Tin Bronze
- Main Composition: Copper with a core alloying element of tin, typically ranging from 4% to 10%. Small amounts of phosphorus are sometimes added.
- Characteristics: High hardness, high strength, good corrosion resistance, and excellent castability.
- Aluminum Bronze
- Main Composition: Copper with a core alloying element of aluminum, typically ranging from 5% to 11%. It may also contain elements like iron and nickel.
- Characteristics: Extremely strong resistance to corrosion and wear, with high strength that can even surpass many stainless steels.
- Silicon Bronze
- Main Composition: Copper with a core alloying element of silicon, typically ranging from 2% to 4%.
- Characteristics: High strength, good ductility, and excellent weldability.
Typical Applications of Bronze
Due to its versatile properties, bronze is utilized across a wide range of industries and applications.
- Industrial Manufacturing: Gears, bearings, bushings, valves.
- Marine Industry: Propellers, pump components, wear-resistant bearings.
- Architecture and Art: Sculptures, decorative items, fasteners.
Conclusion
Bronze is more than just a metal; it is an alloy with both historical significance and modern industrial value. From ancient artifacts to modern precision machinery, bronze’s robust, durable, and castable properties ensure it remains a trusted choice in both engineering and the arts.
If you have any custom bronze casting needs or questions about material selection, feel free to contact our team of experts. We will provide you with professional solutions and support.